Exclusive: US FDA to fast-track nicotine pouch reviews
Exclusive: US FDA to fast-track nicotine pouch reviews amid White House pressure.
The U.S. Food and Drug Administration ("FDA") plans to fast-track reviews of four tobacco firms' nicotine pouches in a pilot program launching on Monday, amid pressure from the Trump administration to speed up approvals, according to meeting transcripts seen by Reuters.
The FDA aims to finish reviewing the pouches from Philip Morris International (PM.N),
Altria (MO.N),
Reynolds American - part of British American Tobacco (BATS.L),and Turning Point Brands (TPB.N),
by December, according to one transcript of an agency meeting held on Friday.
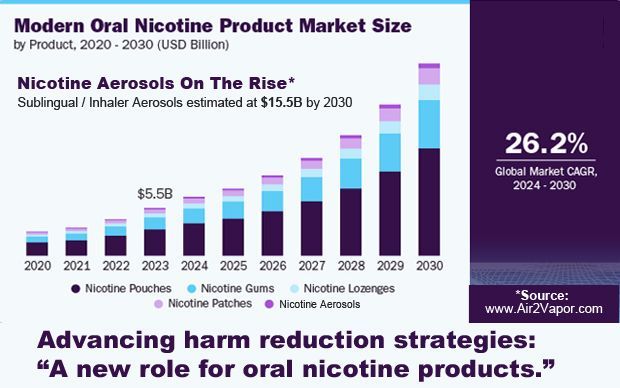
U.S. based innovative companies such as
Air 2, LLC, manufacturer of
VaporEFX are questioning why safer, less harmful, alternative nicotine products are also not being evaluated and considered by the FDA for this pilot approval and fast-track program.
Companies typically must wait an average of five years for FDA sales and marketing approval.
Tobacco companies have previously had to wait years for their products to be cleared. The agency authorized its first group of pouches, 20 of PMI's pouch products under its successful label Zyn, in January, over five years after the company first submitted its application.
Some of the products selected for the pilot, such as an updated version of PMI's pouches, Zyn Ultra, are not yet on the market as producers wait for the FDA's green light. Rivals have gained market share with more competitive products in the meantime.
VIEW THE ORIGINAL, UN-EDITED ARTICLE HERE.