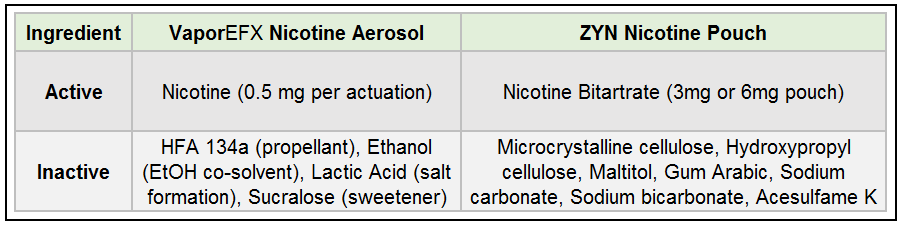
VaporEFX Sublingual Nicotine Aerosol vs. ZYN Nicotine Pouch
Product Overview
VaporEFX
vs ZYN
VaporEFX Sublingual/Oral Nicotine Aerosol:
A novel micellized liquid nicotine delivery system (developed by Air2) that uses a pressurized metered-dose aerosol for oral administration. The aerosol is sprayed into the mouth (sublingually or lingually), delivering nicotine for absorption through the oral mucosa. Each actuation is formulated to release a precise dose (e.g. ~0.5 mg nicotine per each aerosol spray of formulation).
ZYN Oral Nicotine Pouch:
A commercially available smokeless nicotine product (by Swedish Match/Philip Morris International) in the form of a small pouch placed between the gum and lip. It delivers nicotine salt slowly through the gum(s) over a 10-60 minute period. ZYN pouches come in fixed nicotine strengths (typically 3 mg or 6 mg of nicotine per pouch).
Ingredients and Formulation
VaporEFX
vs ZYN
Active Ingredients (Nicotine):
- VaporEFX Sublingual:
Nicotine free base is converted to a nicotine lactate salt in the formulation (using lactic acid). Nicotine lactate is more stable and less volatile than free nicotine, facilitating smoother delivery. Strength per actuation is ~0.5 mg nicotine in the oral aerosol (higher than the inhaler version due to oral route requirements).
- ZYN Pouch:
Nicotine is present as a nicotine salt – specifically nicotine bitartrate dihydrate (a pharmaceutical-grade nicotine salt). Each pouch contains a fixed total amount of nicotine (3 mg or 6 mg); the user’s absorption will occur gradually while the pouch is in contact with the gum.
Inactive Ingredients (Excipients):
- VaporEFX Sublingual:
- Hydrofluoroalkane, (HFA 134a 1,1,1,2-tetrafluoroethane): A propellant that expels the formulation as a fine aerosol spray.
- Ethanol: Used as a co-solvent. Ethanol facilitates the miscibility of nicotine with the propellant and ensures consistent spray uniformity.
- Lactic Acid: An acidifier that converts free-base nicotine to nicotine lactate (salt). This improves nicotine stability and reduces volatility.
- Sucralose: A non-caloric sweetener added to mask nicotine’s bitterness and improve palatability of the aerosol mist.
- ZYN Pouch:
- Stabilizer: Hydroxypropyl Cellulose (plant-derived polymer) to maintain pouch texture.
- Fillers: Microcrystalline cellulose, maltitol, and gum arabic, which add bulk and structure to the pouch matrix. These ingredients are common food additives (cellulose is a plant fiber; maltitol is a sugar alcohol; gum arabic is a natural gum) and help control release of nicotine.
- pH Adjusters: Sodium carbonate and sodium bicarbonate (mineral buffers) to create an alkaline environment in the pouch. This raises the pH, which facilitates conversion of nicotine salt to free-base nicotine for more efficient absorption in the mouth. (However, a higher pH can irritate mucosal tissues, as discussed below).
- Sweetener: Acesulfame potassium (“Ace-K”), an intense artificial sweetener used to impart a slight sweetness.
VaporEFX
Sublingual vs
ZYN Oral Pouch
Summary of Key Formulation Differences
The VaporEFX is a liquid formulation delivered via a metered-dose canister, whereas ZYN is a solid pouch matrix. The aerosol’s excipients (HFA 134a, ethanol, lactic acid, sucralose) are pharmaceutical-grade and enable a rapid, metered dose to the oral mucosa. In contrast, ZYN’s excipients (cellulose, maltitol, etc.) are food-grade components designed for a slow, sustained nicotine release in the mouth. Notably, VaporEFX aerosol uses a propellant and co-solvent to create an instant mist, while ZYN relies on saliva to gradually release nicotine from the pouch over time.
Safety of Excipients and Maximum Daily Exposure (MDE) Considerations.
Both products use
inactive ingredients that are generally recognized as safe, but their safety profiles and exposure limits can differ due to route and amount of exposure. Below is an analysis of the excipients, using FDA and Handbook of Pharmaceutical Excipients data, focusing on safety and any known maximum daily exposure (MDE) or acceptable intake values:
- HFA 134a (Propellant in VaporEFX Aerosol):
This propellant is pharmacologically inert at the levels used in inhalation products. HFA-134a is considered very safe as an inhalation excipient, with a wide safety margin. - Ethanol
(in VaporEFX Aerosol): Ethanol is present as a minor excipient in the aerosol. Each actuation releases only a tiny amount of alcohol – on the order of a few milligrams. Ethanol exposure from the aerosol is negligible and well within safe limits.
- Lactic Acid (in VaporEFX Aerosol): Lactic acid is used to form the nicotine lactate salt.
The FDA affirms lactic acid as GRAS (Generally Recognized as Safe) for use as a direct food additive with no limitations other than good manufacturing practice.
This means in foods (and by extension oral consumption) there isn’t a specific numeric MDE; it can be used at levels necessary to achieve its function, as long as those levels are within normal usage. The amount of lactic acid per aerosol spray is very small – roughly equimolar to nicotine. For example, 0.5 mg of nicotine (as free base) corresponds to about 0.3–0.4 mg of lactic acid to form the salt.
- Safety: Lactic acid in small quantities is very safe; No adverse effects are expected at the levels used in the Air2 product. ZYN does not use lactic acid; instead, it uses carbonate buffers to manipulate nicotine form. Sodium carbonate/bicarbonate are also GRAS food additives, but they create an alkaline pH which can irritate mucosa with prolonged contact. There is no strict MDE for these buffers either – they are safe in the small amounts in a pouch, on the order of a few tens of milligrams – but their local effect is more relevant, as discussed under Irritation below. No adverse effects are expected at the nicotine levels used in the Air2 products.
- Sucralose (in VaporEFX Aerosol): Sucralose is a high-potency non-nutritive sweetener. It is approved as a food sweetener and has an established Acceptable Daily Intake (ADI) of 5 mg per kg body weight per day by the FDA (and up to 15 mg/kg/day by some international authorities). For a 70 kg adult, 5 mg/kg is 350 mg of sucralose per day considered safe for a lifetime. The amount of sucralose in each nicotine aerosol spray is extremely low – likely well under 1 mg – just enough to lightly sweeten the spray. The maximum daily exposure in practice is well below the established safe threshold. Moreover, sucralose is not metabolized into harmful substances in the body; whereas it passes through or is excreted naturally.
- Excipients in ZYN Pouch:
- Hydroxypropyl Cellulose (HPC) and Microcrystalline Cellulose: These are inert polysaccharide-based excipients. They are not absorbed and have no significant toxicity – effectively forms of dietary fiber. There is no specific daily exposure limit; they are used in many oral products. Safety is high, though consuming extremely large amounts of cellulose could cause digestive upset, the amount in pouches (a few hundred milligrams at most) is harmless.
- Maltitol: A sugar alcohol sweetener/filler. It is GRAS (Generally Recognized as Safe) for foods, but excessive oral intake of sugar alcohols can cause laxative effects. The quantity in one pouch is small (maybe on the order of tens of milligrams), which is far below the threshold that causes any GI effects. No specific MDE from FDA, but as a food additive it’s considered safe; just anecdotally if someone used an extreme number of pouches, the cumulative maltitol could cause minor stomach discomfort, though this is unlikely in normal use.
- Gum Arabic: A natural acacia tree gum, used as a binder/thickener in foods (e.g. in chewing gum and candies). It’s safe and GRAS. Large doses can cause mild gas or allergies in rare cases, but in pouch amounts it’s benign.
- Acesulfame K (Ace-K): An FDA-approved sweetener with an ADI of about 15 mg/kg/day. Each pouch contains a tiny fraction of that (likely a few milligrams or less). Even the heavy use of pouches would not exceed safe daily intake for Ace-K. Ace-K is not known to cause oral health issues at such low levels, and it’s not metabolized into harmful products.
Summary:
In summary,
both products use excipients within safe exposure limits. The VaporEFX aerosol excipients are typical of pharmaceutical inhalation/oral products and are used at minimal doses, supported by regulatory safety assessments (ethanol and HFA in many inhalers, sucralose and lactic acid in food/Pharma with wide safety margins). ZYN’s excipients are common food additives also used well within safe limits per pouch. Therefore,
from a systemic toxicity standpoint, the ingredients in both the VaporEFX aerosol and ZYN pouch are expected to be very low risk at the amounts delivered.
Performance and Safety Aspects: Sublingual Aerosol vs. Nicotine Pouch.
Beyond ingredient safety, the
route of delivery and contact time lead to different performance and safety profiles for the VaporEFX aerosol versus the ZYN pouch:
- Micellized Liquid Nicotine Formula: The VaporEFX sublingual utilizes a micellized liquid nicotine formulation that enhances the miscibility and bioavailability of nicotine absorption relative to nicotine pouches. This allows users to achieve equivalent satisfaction with a reduced nicotine dose, providing a clear advantage for VaporEFX over nicotine pouches and other forms of oral or mucosal nicotine delivery.
- Nicotine Delivery Speed: The VaporEFX aerosol provides a rapid release of nicotine into the mouth in a single quick spray. Nicotine lactate from the aerosol will begin to absorb through the lining of the mouth within minutes. This is somewhat analogous to nicotine mouth sprays (like Nicorette QuickMist), which can produce a faster nicotine spike than lozenges. The ZYN pouch releases nicotine much more slowly – where the user typically keeps the pouch in the same place for up to an hour and nicotine is steadily released and absorbed during that time. This slower, steady release may result in a more gradual rise in blood nicotine levels compared to the faster onset from an aerosol spray. For a user seeking quick craving relief, the aerosol has an advantage in onset speed; the pouch provides a longer-lasting, slow burn of nicotine delivery.
- Dose Control and Nicotine Strength: Each actuation of the VaporEFX is a metered dose (~0.5 mg nicotine). Users can titrate their nicotine intake by choosing how many sprays to take (like how smokers control puffs, or how one might take 1–2 sprays of a medicinal nicotine mouthspray). In contrast, a ZYN pouch has a fixed total amount of nicotine (3mg or 6 mg) that will be released; users can’t take “half a pouch;” they must remove it to reduce the amount of nicotine absorbed. In practice, most will use one pouch at a time. The pouch delivers more nicotine overall per unit (even a “low” 3 mg pouch exceeds one aerosol spray) but spread out over time. From a performance perspective, the aerosol delivers an immediate nicotine peak per dose, whereas the pouch yields a sustained plateau of nicotine.
- Oral Contact and Irritation: A key difference is how long the product’s contents are in contact with the oral mucosa:
- VaporEFX: The aerosol spray distributes nicotine onto the mouth and likely some is swallowed. The contact time is relatively brief – the liquid is quickly absorbed or swallowed within seconds. The formulation’s pH is likely near neutral (nicotine lactate would make it slightly acidic). Because of the short contact and lack of high alkalinity, the aerosol is unlikely to cause significant local irritation.
- ZYN Pouch: The pouch is held against the gum for many minutes, which is prolonged exposure. The ingredients in the pouch (especially the nicotine itself and the high pH from sodium carbonate/bicarbonate) can irritate the oral mucosa with chronic use. Nicotine is a vasoconstrictor and can reduce blood flow to gum tissue, and the alkaline pH (needed to freebase the nicotine) can cause a burning or tingling sensation.
Moreover, users often report that long-term use of nicotine pouches leads to localized gum issues. Indeed, mouth sores, gum irritation, and even ulcers have been reported with frequent nicotine pouch use. Continuous exposure to the pouch in one spot can lead to gum line recession or soreness in that area. ZYN’s own packaging includes warnings about potential mouth irritation. In summary, oral irritation is a known risk with nicotine pouches, whereas the Air2 aerosol likely mitigates this risk by delivering nicotine rapidly without prolonged contact or high pH in the mouth.
- Systemic Side Effects: Nicotine’s systemic effects (increased heart rate, blood pressure, potential dizziness or nausea in new users) will be present with both products, as both deliver pharmacologically active nicotine. However, the peak nicotine levels and their timing might differ. A fast spike (from the aerosol) could theoretically produce a quick “rush” or, for some, slightly more pronounced transient effects (like a light-headed feeling), whereas the pouch’s slower delivery might cause more subtle onset. That said, both are delivering nicotine in amounts that are within the range of nicotine replacement therapies or tobacco products. It’s worth noting that nicotine in any form is addictive and not risk-free: it can contribute to hypertension and other cardiovascular effects over time. Neither product is risk-free for a non-smoker; they are intended for adult nicotine users. The advantage of these products over smoking is the absence of combustion products (no tar, carbon monoxide, etc.), which dramatically reduces many health risks. But users should be aware that nicotine itself can have health impacts (like on heart health and potential insulin resistance, etc., though those are far less severe than the risks of smoking).
- Potential for Adverse Events from Excipients: In the VaporEFX aerosol, as discussed, excipients are minimal and used in many medications. A very small subset of individuals could be sensitive (for example, a rare alcohol hypersensitivity or an allergy to sucralose, which is exceedingly uncommon). ZYN’s excipients could theoretically cause minor issues: e.g., some people might be sensitive to a particular flavor or sweetener (acesulfame K sensitivity is rare but possible). The maltitol could cause a bit of stomach upset if someone uses many pouches and swallows the residue, but this would require heavy use. Generally, both products’ non-nicotine ingredients are well-tolerated by most users at these doses.
In conclusion, the VaporEFX oral nicotine aerosol and the ZYN nicotine pouch both aim to deliver nicotine without smoking, but they differ substantially in formulation and delivery mechanism. The VaporEFX aerosol offers rapid, metered dosing with pharmaceutical excipients and no prolonged oral contact, potentially reducing risks of irritation and eliminating any need for combustion or heat.
The ZYN pouch provides slow-release, longer nicotine experience with all food-grade ingredients, but users should be aware of possible gum irritation with extended use.
In other words, Air 2 aerosol's rapid, controlled nicotine delivery and minimal nicotine exposure offer clear safety advantages over prolonged ZYN pouch use.
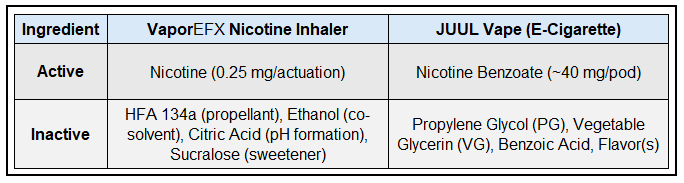
VaporEFX™ Nicotine Inhaler vs. JUUL Vape (E-Cigarette)
Product Overview
VaporEFX
vs JUUL
VaporEFX Nicotine Inhaler:
A propellant-driven nicotine inhaler (developed by Air2) that delivers nicotine aerosol for inhalation. Each actuation releases a measured dose (e.g. ~0.25 mg nicotine per spray in the inhaler formulation). The user inhales the spray, sending nicotine particles into the respiratory tract for rapid absorption (similar speed to smoking, since the lungs provide a quick route to the bloodstream). Importantly, the Air2 inhaler operates without any heating or combustion and relies on pressure from the propellant to create an aerosol.
JUUL Vape (E-Cigarette):
JUUL is a popular electronic cigarette device (vape) that delivers nicotine via a battery-powered heating element. JUUL consists of a rechargeable device and disposable pods containing nicotine e-liquid. When the user draws on the device, the liquid is heated by a coil to produce an inhalable vapor. JUUL’s nicotine is a high-strength nicotine salt formulation (nicotine benzoate). A standard JUUL pod contains ~0.7 mL of e-liquid at 3% or 5% nicotine by weight – equating to about 23–40 mg of nicotine per pod.
This is roughly comparable to the nicotine in a pack of cigarettes (JUUL Labs noted ~40 mg in a 5% pod and approximately one pack of cigarettes nicotine-wise). JUUL delivers nicotine to the lungs rapidly through inhalation of the warm aerosol, and its nicotine salt formulation makes the vapor less harsh despite high nicotine levels.
Ingredients and Formulation
VaporEFX
vs JUUL
Active Ingredients (Nicotine):
- VaporEFX Inhaler:
Nicotine is delivered as nicotine citrate. The nicotine dose per actuation is ~0.25 mg (half the strength of the oral version, since lung absorption is very efficient). The micellized nicotine formulation is a solution in propellant creating a homogeneous mixture, allowing nicotine to be aerosolized.
- JUUL Vape (POD):
Contains nicotine in the form of nicotine benzoate (nicotine salt with benzoic acid). JUUL’s proprietary e-liquid formula combines freebase nicotine with benzoic acid to create a nicotine salt; this allows a high concentration (3–5% nicotine) while maintaining smoothness on the throat. When vaporized, this yields an aerosol rich in nicotine particles that are readily absorbed in the lungs.
Inactive Ingredients:
- VaporEFX Inhaler Excipients:
- The inhaler formula is very similar to the VaporEFX sublingual (except the higher nicotine content). It includes:
- HFA 134a Propellant:
- (Same as described earlier) The compressed gas that propels and atomizes the dose. Provides the force to deliver nicotine particles deep into the lower respiratory tract.
- Ethanol: Co-solvent to help nicotine being miscible with the propellant and aid aerosol formation. Present in small quantity per spray.
- Citric Acid: Added to convert nicotine to the citrate salt, improving stability and inhalation smoothness.
- Menthol: Added as a flavoring agent.
- Note: The VaporEFX inhaler formulation contains no glycerin, propylene glycol (PG), or other typical e-cig additives. It delivers a pure nicotine aerosol without generating new chemicals via heat.
- JUUL Vape (POD) Excipients:
- Propylene Glycol and Vegetable Glycerin (VG): These two chemical compounds make up the bulk of the e-liquid. JUUL uses a mixture (approximately 30% PG and 70% VG in published analyses). PG is a carrier that produces vapor and carries flavor, while VG is a thicker liquid that creates dense vapor clouds. Both PG and VG are common in all e-cigarettes as the primary vaporization media.
- Benzoic Acid: As noted, benzoic acid is added to form nicotine benzoate salt. It also serves as a mild preservative. The exact concentration isn’t publicly listed, but it’s a key component. Benzoic acid in JUUL likely constitutes a few percent of the liquid. It helps make the nicotine hit tolerable (reducing harshness) and may slightly affect pH to optimize nicotine absorption.
- Flavoring agents: JUUL pods contain flavor chemicals to produce their tobacco or menthol. The specific flavor compounds are proprietary and not disclosed on the package. They could include various FDA-approved flavoring agents used in food. Notably, JUUL’s current lineup avoids certain flavors like creme or fruit in compliance with flavor restrictions. Menthol and tobacco flavors still contain some cooling or aromatic compounds. These flavor chemicals are dissolved in the PG/VG base.
- (No Added Sweetener listed): Unlike some aftermarket e-liquids that add sweeteners like sucralose or ethyl maltol, JUUL has not advertised any explicit sweetener in their formula. However, some flavors might impart sweetness (e.g. ethyl maltol is a cotton-candy sweet flavor used in some tobacco e-liquids). It’s unclear if JUUL uses it, but one study did note JUUL’s flavor could include such compounds
VaporEFX
Inhaler
vs JUUL Vape
Summary of Key Formulation Differences
The VaporEFX nicotine inhaler is a pharmaceutical-style formulation with minimal ingredients: Micellized nicotine, an organic acid (lactic), an FDA approved propellant, and trace Ethanol and sweetener – no glycerol or large organic solvents. JUUL’s e-liquid is a complex mixture of organic solvents (PG/VG) and flavor chemicals that must be heated to create vapor. JUUL delivers a much larger reservoir of nicotine (a pod has tens of mg nicotine; an inhaler actuation has <1 mg). The VaporEFX inhaler provides precise, metered doses, whereas JUUL provides a continuous spectrum of doses controlled by how long and how often the user inhales the JUUL Vape product.
Safety of Excipients and Maximum Daily Exposure (MDE) Considerations.
Examining the inactive ingredients in the Air2 inhaler vs. JUUL Vape (E-Cigarette), focusing on safety profiles and any known exposure limits:
- HFA 134a (Propellant in VaporEFX Inhaler):
This propellant is pharmacologically inert at the levels used in inhalation products. HFA-134a is considered very safe as an inhalation excipient, with a wide safety margin. It has no significant pharmacological effect at the doses from inhalers, and it is eliminated rapidly. The use of HFA 134a in the nicotine inhaler is not a safety concern. JUUL: no propellant used; instead uses battery power. - Ethanol
(in VaporEFX Inhaler): The inhaler’s ethanol content per puff is, like the oral spray, on the order of <10 mg. Ethanol as an excipient in the inhaler poses no intoxication risk and has been used safely in many inhalers for solubility. JUUL’s e-liquid does
not contain alcohol/ethanol as a listed component, so ethanol exposure is not relevant for JUUL.
- Citric Acid (in VaporEFX Inhaler): The citric acid creates nicotine citrate; again, the quantity per puff is very small (fractions of a milligram). Being GRAS in foods with no strict limits, and commonly used in pharmaceuticals, this amount is harmless. Importantly, by forming salt, lactic acid helps avoid the irritation that free nicotine base might cause. No specific daily limits have been established. JUUL: Uses
benzoic acid (not lactic acid); see details provided below. Important note: Citric acid is listed within the FDA’s Inactive Ingredient Database (IID) as approved for inhalation/pulmonary use.
- Safety: Citric acid in small quantities is very safe; No adverse effects are expected at the levels used in the Air2 product. JUUL does not use lactic acid; instead, it uses carbonate buffers to manipulate nicotine form. Sodium carbonate/bicarbonate are also GRAS food additives, but they create an alkaline pH which can irritate mucosa with prolonged contact. There is no strict MDE for these buffers either – they are safe, within a few tenths-of-milligrams, but their local effect is more relevant, as discussed under Irritation below.
- Menthol (in VaporEFX Inhaler): Included for taste. The safety discussion is similar to above: ADI 5 mg/kg/day, and actual exposure from the inhaler is far below that. No known issues exist with an inhaled dose of Menthol because the amount is small and the formulation is not heated. JUUL: Some studies note many sweetened e-liquids use sucralose or other sweetening agents; JUUL’s sweetness mostly comes from glycerin’s sweet character. If JUUL did contain a sweetener, the amounts would also likely be low – but since not confirmed, we focus on known components.
Excipients in JUUL Vape (E-Cigarette):
Propylene Glycol (PG) in JUUL: PG is Generally Recognized as Safe (GRAS) for use in food and also used in medicines (including some inhaled medications like nebulizer solutions). There is no formal FDA “maximum daily intake” for PG in inhalation, but toxicological guidance exists.
Safety: PG inhalation can cause mild irritation of the airways in some people. Studies and experience show that PG can dry out throat and might cause a slight throat hit. Importantly, while PG is safe to eat, inhalation safety is not fully the same. Some research indicates that inhaling PG/VG aerosols can irritate the lung lining and may trigger inflammatory changes. For example, a study found that e-cig users’ airways had changes in immune protein expression consistent with irritation after exposure to PG/VG aerosols. However, no acute toxicity occurs at typical vaping levels – it’s more about potential chronic effects. MDE: There isn’t a defined MDE for inhaled PG by regulators, but e-cigarette studies so far have not identified a specific “toxic dose” in humans – the main concern is long-term exposure. In summary, PG is relatively safe but not completely inert in the lungs: it can cause some irritation, and its long-term high-dose inhalation effects are still under study.
Vegetable Glycerin (VG) in JUUL: VG (glycerol) is also GRAS for oral use, used in foods and cosmetics. It’s generally regarded as benign. In vaping, VG produces the visible cloud. Like PG, inhaled glycerin can cause mild airway irritation or dryness. Glycerin vapor at high temperature can decompose to produce acrolein, a respiratory irritant, if the device overheats. Under normal JUUL operating conditions (regulated temperature), formaldehyde or acrolein yields are low, but not zero – especially if a pod runs dry or someone uses high power (less an issue in JUUL which has controlled power). Exposure: If a pod is ~70% VG, that’s ~0.5 mL ≈ 630 mg glycerin per pod. Again, within oral safe intake (glycerin is used as a sweetener/laxative sometimes, oral doses of grams are tolerated). Inhalation, the body can handle some, but excessive glycerin inhalation might lead to a heavy feeling in chest for some users. No formal MDE, but likely safe in the hundreds of mg range per day based on current knowledge. Safety note: Chronic inhalation of glycerol hasn’t shown severe disease in users so far, but research is ongoing. It’s considered of low acute toxicity, with the main cautions being the byproducts of heating rather than glycerin itself.
Benzoic Acid (JUUL): Benzoic acid is a common food preservative (E210) found in foods like soda (as sodium benzoate). It has an established ADI ~5 mg/kg/day (so ~350 mg for a 70 kg adult). JUUL pods likely contain on the order of a few percent benzoic acid. Safety: In the vapor, benzoic acid mostly stays in salt form with nicotine; some of it might deposit in the throat/lungs. It might contribute to throat irritation if any free benzoic acid is present (benzoic acid is a mild irritant at higher concentrations). However, no major toxicity is expected at JUUL levels. The main unknown is whether chronic inhalation of nicotine benzoate has any unique effects – one article noted the health effects of inhaling nicotine in salt form with benzoic acid are not fully known yet. So, while benzoic acid is safe as a food additive, inhalation toxicology is less studied. But given the small quantities, experts currently believe it’s low risk.
- Flavor (JUUL): This is a broad category. Specific flavoring agents in e-cigs can include compounds like menthol (in menthol pods), various esters, aldehydes, etc. Many of these are FEMA GRAS for flavor use in food, but inhalation safety can differ. For instance,
diacetyl, a butter flavor chemical, is safe to ingest but
can cause “popcorn lung” (bronchiolitis obliterans) when inhaled chronically. The American Cancer Society has cautioned that some e-cig flavors (especially buttery or creamy ones) may contain diacetyl linked to lung damage. JUUL in particular stated they do not use diacetyl in their flavors, especially after this became known. Their current menthol and tobacco flavors likely rely on menthol, eucalyptol, and other flavors considered less harmful. Still,
the inhalation safety of flavor chemicals is an area of concern – certain components can be irritants or sensitizers. For example, cinnamon flavor (cinnamaldehyde) is known to be cytotoxic to lung cells in vitro; we don’t believe JUUL uses cinnamon in current pods, but it’s an example of a risky flavor.
Exposure limits: There are no set inhalation limits for these flavorings; the industry relies on the principle that if they are used at low concentrations and not overtly toxic, they’re “acceptable.” It’s a bit of a grey area, and research is ongoing to identify which flavor chemicals are safest. In summary, flavorings are a necessary part of e-cig appeal but represent a potential
unknown risk factor (the fewer and simpler the flavor chemicals, generally the better).
- Metals and Impurities: This isn’t an “ingredient” per se, but it’s critical for safety. E-cigarette users can be exposed to
metallic particles and other contaminants from the device itself. The heating coil in JUUL (and other vapes) is metal (often nichrome or similar). Studies have found that e-cig aerosol can contain
toxic metals like nickel, chromium, lead, and others, likely shed from the coil or metal components. Notably, a Johns Hopkins study (Rule, 2018) found measurable levels of metals in both the e-liquid (even before heating) and the aerosol of e-cigs. These metals, albeit in small quantities, are
inhaled directly to the lungs. Metals such as nickel and chromium are known inhalation carcinogens when exposure is high or prolonged. While the levels in a single JUUL puff are extremely low, chronic daily vaping could cumulatively increase exposure to these metals. There are no set exposure limits for these metals in e-cig vapor yet, but their presence is concerning. By contrast, the Air2 inhaler, being a sealed canister with pharmaceutical-grade components, is
highly unlikely to introduce metal nanoparticles – it has no heating coil and no metal in contact with the formulation (MDI canisters are usually aluminum, but the propellant and drug are not in contact with heating or abrasive processes that release metal). Thus,
the Air2 inhaler avoids the issue of heavy metal exposure that some e-cigarette users face.
- Impurities and Contaminants in Liquids: Pharmaceutical-grade products like the Air2 inhaler are made to high purity standards (GMP), so the risk of unknown impurities (e.g., residual solvents, harmful contaminants) is minimal and monitored. On the other hand, in the broader vaping market, there have been serious issues with adulterants. The most dramatic example was the 2019 outbreak of
EVALI (E-cigarette or Vaping-Associated Lung Injury). Over
2,800 hospitalizations and 68 deaths were reported in the U.S. from a severe lung illness linked to vaping. Investigations pointed to
vitamin E acetate, an oil-like additive used illicitly in some THC (and possibly some nicotine) vape products, as a key culprit. When inhaled, vitamin E acetate can disrupt lung function and triggered acute respiratory failure in many cases. While JUUL pods were not found to contain vitamin E acetate (the problem was mainly in black-market THC carts), this highlighted how
impurities or additives in vapor products can cause severe toxicity. Even outside of EVALI, some nicotine e-liquids have had quality control issues (e.g., contamination with diethylene glycol in early e-cigs was once reported).
The Air2 inhaler is expected to have a more consistent purity and safety profile with regard to contaminants, whereas JUUL and other e-cigs rely on manufacturer quality.
Summary on Excipient Safety:
The VaporEFX inhaler’s excipients (HFA 134a, Ethanol, Citric acid and Menthol) each have strong safety records in their domains and are used in controlled, small quantities – well within any known exposure limits. JUUL’s ingredients (PG, VG, benzoic acid, flavors) are mostly GRAS for ingestion and likely safe in moderate inhalation exposure,
but they do carry more unknowns. PG and VG can irritate airways; certain flavor chemicals might pose risks; and the heating process can introduce trace harmful byproducts (like formaldehyde, acetaldehyde, Acrolein, metal particles) that simply do not exist in the Air2 inhaler’s output.
Performance and Safety Aspects: Inhaler Aerosol vs. Vape (E-Cigarette).
Beyond ingredient safety, the
route of delivery and contact time lead to different performance and safety profiles for the VaporEFX Inhaler versus the JUUL Vape:
- Nicotine Delivery and Pharmacokinetics: Both the Air2 inhaler and JUUL aim to deliver nicotine to the lungs, which is the fastest route to the bloodstream aside from intravenous. Nicotine reaching the lung alveoli is absorbed within seconds, leading to a rapid rise in arterial blood nicotine – closely mimicking the spike from a traditional cigarette.
- The Air2 inhaler delivers a
metered 0.25 mg per puff. If a user takes, say, 4 puffs, that’s ~1 mg nicotine, roughly akin to one cigarette’s worth of nicotine absorbed (a typical cigarette yields ~1–2 mg to the smoker’s bloodstream). The dosing is precise and known per actuation.
- JUUL’s delivery per puff is less uniform: A user might get about 0.1 to 0.2 mg nicotine per puff (depending on puff volume and duration). JUUL’s 5% pods are designed to deliver cigarette-like nicotine levels efficiently; indeed,
users get more nicotine per puff with JUUL than with many other e-cigs. A full JUUL pod (40 mg nicotine) could theoretically equal the nicotine in 20 cigarettes, but not all nicotine is absorbed – still, it’s potent. Many users finish a pod in a day or two, meaning an intake of perhaps 20–40 mg nicotine per day, similar to a pack-a-day smoker.
- Onset: Both products give a quick hit, the Air2 inhaler’s nicotine is in citric acid form (slightly acidic aerosol) whereas JUUL’s is in benzoate form (slightly acidic aerosol as well). Both are designed to be smooth and not harsh on the throat/lung (freebase nicotine at these levels would be very harsh due to alkalinity, but the salts make inhalation comfortable). The net result is that either device can satisfy cravings rapidly. However, the Air2 inhaler might deliver a measured pulse of nicotine with each spray, whereas JUUL users might take a long drag yielding perhaps a larger dose if they choose. In terms of user behavior the inhaler might lend itself to a more “medical” usage pattern (a fixed number of puffs at certain times), whereas JUUL can be sipped or heavily vaped at the user’s discretion, potentially leading to higher overall intake if one isn’t cautious.
- Consistency and Dosing Control: A notable benefit of the Air2 inhaler is dosing consistency. Each actuation is controlled. There’s no worry about puff duration or device power – the canister releases the same dose each time. This can help avoid excessive nicotine intake. With JUUL, although the device regulates power to some extent, the user can still inadvertently intake a lot of nicotine by taking many puffs in succession. There have been reports of inexperienced users getting
nicotine sickness (nausea, dizziness) from chain-vaping high-nicotine pod systems like JUUL, because it’s easy to take many puffs quickly. The Air2 inhaler, being more akin to a medicine, may encourage more mindful dosing (plus it delivers fewer puffs per container – e.g., an inhaler might have 100–200 doses in a canister, whereas a JUUL pod can provide perhaps 200+ puffs). In short,
the inhaler is metered, JUUL is ad libitum – this influences the potential for misuse.
- Thermal Decomposition and Toxic Byproducts:
This is one of the biggest differences in safety:
- The Air2 inhaler uses no heat. The nicotine and excipients are not exposed to high temperatures. They remain chemically stable (nicotine lactate will not decompose at ambient temperature; HFA and ethanol evaporate but do not “burn” anything). Therefore, the aerosol from Air2 is essentially the same substances that went in: nicotine, lactic acid, Ethanol, and sucralose – all just dispersed into tiny droplets or particles. No new chemicals are generated in the process.
- JUUL’s operation involves heating the e-liquid to around ~200-250° C (estimated, as JUUL’s voltage and coil design produce a certain temperature range). This heating causes
vaporization of PG, VG, nicotine, etc., but it can also cause
thermal decomposition of some components:
- Heating e-liquids for vaping is known to be dangerous because it creates toxic chemicals like formaldehyde and acetaldehyde, which are linked to cancer and DNA damage. Inhaling these substances can cause lung irritation and tissue damage, leading to chronic respiratory diseases.
- PG can oxidize to propylene oxide or break into small aldehydes like formaldehyde when overheated.
- VG can dehydrate to acrolein (a lung irritant) and also produce formaldehyde or acetaldehyde at high temperatures.
- Nicotine itself can oxidize to nicotine-related impurities (though major decomposition is less at these temps, some minor products can form).
- Flavor chemicals can break down or react with PG/VG. For example, sweet flavors can form acetals or other compounds in vapor.
- Even benzoic acid at high temperature might decarboxylate or form benzene in trace amounts (there’s some research showing very slight benzene formation in some e-cig vapors, likely from benzoate or other aromatic compounds at high power).
- In normal use, JUUL’s temperature control avoids the coil getting red-hot (which is good, it prevents a lot of toxic byproducts). But if a device malfunctions or a user deliberately modulates it (though JUUL is closed-system, so less user tinkering), these decomposition products could increase.
- There have been experiments measuring carbonyl compounds (formaldehyde, etc.) in e-cig vapor: when devices are used properly (“wet” coils), these compounds are relatively low, but in “dry puff” conditions they can spike. JUUL’s design mostly prevents dry puffs by capillary wick and regulated power, but not entirely foolproof if chain vaped.
- By contrast, the Air2 inhaler produces none of these thermal degradants – its aerosol is “cold.” This is a significant safety advantage: no formaldehyde, acrolein, or other heating byproducts that are known to contribute to the risks of vaping.
- Additionally,
no combustion = no carbon monoxide, no tar etc., for both devices. But vaping still shares some similarities with smoking in terms of the presence of some irritant chemicals (just far fewer and in smaller quantities than cigarettes). The inhaler avoids virtually all of those except nicotine itself.
- Addiction and Abuse Potential: Both products contain addictive nicotine. One could argue JUUL’s high nicotine content and flavorful delivery led to a wave of youth usage and high abuse potential. The Air2 inhaler, being a therapeutic format, might be less enticing to non-smokers. This could be a societal safety advantage – less likely to be misused by teens, for instance. JUUL’s easy concealability and pleasant flavors (at least initially, like mango, etc.) made it a vector for nicotine initiation in young people. In the comparison,
from an abuse-deterrent standpoint, the inhaler is likely more utilitarian (similar to how nicotine inhalers or sprays for quitting are rarely abused by non-smokers), whereas JUUL as a lifestyle product had higher appeal beyond existing smokers. This isn’t a chemical safety point, but it is relevant to overall “safety” in terms of public health impact.
- Long-Term Health Considerations:
- The Air2 inhaler, if used as intended (possibly for smoking cessation or harm reduction), would provide nicotine without the myriad of known toxicants of smoking. Long-term use of such an inhaler would likely have similar health profile to long-term use of nicotine gum or patches – nicotine effects plus perhaps some local irritation (though in lung instead of mouth). Nicotine itself, long-term, can contribute to cardiovascular strain (e.g., slightly elevated heart rate/blood pressure chronically) and is not harmless, but it is dramatically safer than inhaling smoke. We don’t expect the inhaler’s excipients to cause any chronic disease (HFA, ethanol, etc., don’t accumulate or cause organ damage at these levels).
- Long-term vaping (JUUL or others) is still being studied. We know it’s much safer than smoking in terms of cancer and lung function (no tar, far fewer carcinogens). However, some studies indicate that chronic e-cig use can cause
mild lung inflammation, changes in lung cells, and possibly increase risk of respiratory infections or asthma exacerbation in some individuals. Vaping has been linked to reports of chronic bronchitis symptoms in some users (cough, mucus) – possibly due to PG/VG irritating the airways. And as noted, certain flavor chemicals or contaminants could pose risks that manifest over long periods.
- There is also cardiovascular concern: nicotine from vaping can acutely raise blood pressure; some studies suggest chronic vapers might have some of the same cardiovascular risk markers as smokers (though likely lower magnitude). It’s hard to separate nicotine’s effect from other factors, but ongoing research is examining if e-cig use correlates with heart or blood vessel issues over years.
- The Air2 inhaler, delivering nicotine in a cleaner form, would have similar cardiovascular considerations (nicotine is nicotine), but absent the potential lung inflammation from PG/VG or other components, it might be even “cleaner” than vaping from a respiratory standpoint.
Key Safety/Performance Takeaways:
- The Air2 nicotine inhaler provides
nicotine in a metered, non-heated aerosol, avoiding the formation of harmful chemicals that result from the high-temperature operation of vapes. Its excipients have high safety margins (propellant, etc.), and it is essentially delivering pharmaceutical-grade nicotine in a clean form. The main risks are nicotine’s inherent effects and the potential for mild throat irritation or a learning curve to use the device.
- The JUUL vape delivers
nicotine in a flavored, heated vapor, which while vastly cleaner than cigarette smoke, still contains
multiple chemical constituents beyond nicotine, some of which are known or suspected to be harmful (even if at much lower levels than cigarettes). There have been real incidents of lung injury largely due to adulterants in the vaping supply chain – a risk minimized in closed systems like JUUL but illustrative of vaping’s potential dangers. JUUL’s high nicotine content also means users must be careful to avoid nicotine overdose symptoms and addiction reinforcement.
- From a
user experience perspective, JUUL’s ease and potentially pleasurable flavors may lead to more continuous use, whereas the inhaler might be used more intentionally (like a medicine) to curb cravings. This difference can affect total nicotine consumption and thus overall health impact (someone mindlessly puffing a JUUL all day might intake more nicotine than someone who takes a few inhaler puffs only when cravings hit).
- Importantly, neither product produces the combustion toxins of cigarettes, so both are far safer alternatives to smoking. But between them, the Air2 inhaler is designed to eliminate as many risk factors as possible (no heat, and no sugar/alcohol byproducts). JUUL, while a revolutionary reduced-harm product for smokers, still carries extra uncertainties due to its complex chemistry in use.
In Summary:
the VaporEFX nicotine inhaler vs. JUUL Vape (E-Cigarette) exemplifies
a precise nicotine delivery vs. a consumer electronic nicotine delivery. The inhaler focuses on precision, purity, and minimizing additional substances, thereby likely offering a superior safety profile (aside from nicotine dependency issues common to both). JUUL provides a highly effective nicotine hit and smoking substitute, but its reliance on heating and multiple liquid ingredients introduces some avoidable risks – including reports of chemical exposure (trace aldehydes, metals) and past incidents of contamination-related lung injuries in the vaping market.
The VaporEFX inhaler’s propellant-driven system avoids those pitfalls, delivering nicotine with no need for heat and with excipients that have well-characterized safety in inhalation. Users and healthcare providers can weigh these differences when considering nicotine delivery options for smoking cessation or harm reduction.