Product Comparisons
VaporEFX™ Sublingual Nicotine Aerosol vs. ZYN Nicotine Pouch
Product Overview
VaporEFX
vs ZYN
VaporEFX Sublingual/Oral Nicotine Aerosol:
A novel micellized liquid nicotine delivery system (developed by Air2) that uses a pressurized metered-dose aerosol for oral administration. The aerosol is sprayed into the mouth (sublingually or lingually), delivering nicotine for absorption through the oral mucosa. Each actuation is formulated to release a precise dose (e.g. ~0.5 mg nicotine per spray in one example formulation).
ZYN Oral Nicotine Pouch:
A commercially available smokeless nicotine product (by Swedish Match/Philip Morris International) in the form of a small pouch placed between the gum and lip. It delivers nicotine salt slowly through the gum over about 30-60 minutes. ZYN pouches come in fixed nicotine strengths (typically 3 mg or 6 mg of nicotine per pouch).
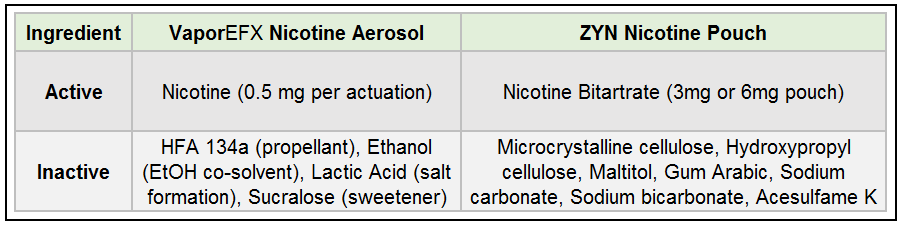
Ingredients and Formulation
VaporEFX
vs ZYN
Active Ingredients (Nicotine):
- VaporEFX Aerosol:
Nicotine free base is converted to a nicotine lactate salt in the formulation (using lactic acid). Nicotine lactate is more stable and less volatile than free nicotine, facilitating smoother delivery. Strength per actuation is ~0.5 mg nicotine in the oral aerosol (higher than the inhaler version due to oral route requirements).
- ZYN Pouch:
Nicotine is present as a nicotine salt – specifically nicotine bitartrate dihydrate (a pharmaceutical-grade nicotine salt)us.zyn.com. Each pouch contains a fixed total amount of nicotine (3 mg or 6 mg); the user’s absorption will occur gradually while the pouch is in contact with the gum.
Inactive Ingredients (Excipients):
- VaporEFX Aerosol:
- HFA 134a (1,1,1,2-tetrafluoroethane): A hydrofluoroalkane propellant that expels the formulation as a fine aerosol spray.
- Ethanol: Used as a co-solvent. Ethanol helps nicotine to being miscible with the propellant and ensure uniform spray.
- Lactic Acid: An acidifier that converts free-base nicotine to nicotine lactate (salt). This improves nicotine stability and reduces volatility.
- Sucralose: A non-caloric sweetener added to mask nicotine’s bitterness and improve palatability of the aerosol mist.
- ZYN Pouch:
- Stabilizer: Hydroxypropyl Cellulose (plant-derived polymer) to maintain pouch texture.
- Fillers: Microcrystalline cellulose, maltitol, and gum arabic, which add bulk and structure to the pouch matrix. These ingredients are common food additives (cellulose is a plant fiber; maltitol is a sugar alcohol; gum arabic is a natural gum) and help control release of nicotine.
- pH Adjusters: Sodium carbonate and sodium bicarbonate (mineral buffers) to create an alkaline environment in the pouch. This raises the pH, which facilitates conversion of nicotine salt to free-base nicotine for more efficient absorption in the mouth. (However, a higher pH can irritate mucosal tissues, as discussed below).
- Sweetener: Acesulfame potassium (“Ace-K”), an intense artificial sweetener used to impart a slight sweetness.
VaporEFX
Sublingual vs
ZYN Oral Pouch
Summary of Key Formulation Differences
The Air2 aerosol is a liquid formulation delivered via a metered-dose canister, whereas ZYN is a solid pouch matrix. The aerosol’s excipients (HFA 134a, ethanol, lactic acid, sucralose) are pharmaceutical-grade and enable a rapid, metered dose to the oral mucosa. In contrast, ZYN’s excipients (cellulose, maltitol, etc.) are food-grade components designed for a slow, sustained nicotine release in the mouth. Notably, Air2’s aerosol uses a propellant and
co-solvent to create an instant mist, while ZYN relies on saliva to gradually release out nicotine from the pouch over time.
Safety of Excipients and Maximum Daily Exposure (MDE) Considerations
Both products use
inactive ingredients that are generally recognized as safe, but their safety profiles and exposure limits can differ due to route and amount of exposure. Below is an analysis of the excipients, using FDA and Handbook of Pharmaceutical Excipients data, focusing on safety and any known maximum daily exposure (MDE) or acceptable intake values:
- HFA 134a (Propellant in VaporEFX Aerosol):
This propellant is pharmacologically inert at the levels used in inhalation products. HFA-134a is considered very safe as an inhalation excipient, with a wide safety margin. - Ethanol
(in VaporEFX Aerosol): Ethanol is present as a minor excipient in the aerosol. Each actuation releases only a tiny amount of alcohol – on the order of a few milligrams. Ethanol exposure from the aerosol is negligible and well within safe limits.
- Lactic Acid (in VaporEFX Aerosol): Lactic acid is used to form the nicotine lactate salt.
The FDA affirms lactic acid as GRAS (Generally Recognized as Safe) for use as a direct food additive with no limitations other than good manufacturing practice.
This means in foods (and by extension oral consumption) there isn’t a specific numeric MDE; it can be used at levels necessary to achieve its function, as long as those levels are within normal usage. The amount of lactic acid per aerosol spray is very small – roughly equimolar to nicotine. For example, 0.5 mg of nicotine (as free base) corresponds to about 0.3–0.4 mg of lactic acid to form the salt.
- Safety: Lactic acid in small quantities is very safe; No adverse effects are expected at the levels used in the Air2 product. ZYN does not use lactic acid; instead, it uses carbonate buffers to manipulate nicotine form. Sodium carbonate/bicarbonate are also GRAS food additives, but they create an alkaline pH which can irritate mucosa with prolonged contact. There is no strict MDE for these buffers either – they are safe in the small amounts in a pouch, on the order of a few tens of milligrams – but their local effect is more relevant, as discussed under Irritation below.
- Sucralose (in VaporEFX Aerosol): Sucralose is a high-potency non-nutritive sweetener. It is approved as a food sweetener and has an established Acceptable Daily Intake (ADI) of 5 mg per kg body weight per day by the FDA (and up to 15 mg/kg/day by some international authorities). For a 70 kg adult, 5 mg/kg is 350 mg of sucralose per day considered safe for a lifetime. The amount of sucralose in each nicotine aerosol spray is extremely low – likely well under 1 mg – just enough to lightly sweeten the spray. The maximum daily exposure in practice is well below the established safe threshold. Moreover, sucralose is not metabolized into harmful substances in the body; it mostly passes through or is excreted.





